Scientific discoveries are key to understanding how the world around us works. Whether it’s the materials and objects or our health and well-being, improving the overall quality of our life would be pretty much impossible without the discoveries in chemistry, physics, and other sciences. And the discovery of the proton is a great example.
Protons are stable subatomic particles with a positive electric charge that were discovered in the early 19th century by Ernest Rutherford.
So, who discovered the proton? Or what’s the difference between protons, neutrons, and electrons? And why are protons so important?
If this is something you’ve been wondering, we’ve got you covered. Here’s everything you need to know about protons, from the discovery to their importance.
Discovery of Proton
In the early 19th century, William Prout developed the concept that chemical elements were composed of hydrogen atoms, called “protyles.” While this seemed to be accurate, further experiments proved that atoms consisted of other subatomic particles rather than hydrogen or hydrogen-like atoms.
In 1897, British physicist Joseph John Thomson shared his discovery of smaller subatomic particles that he originally referred to as “corpuscles,” today known as electrons.
23 years later, in 1919 or 1920, New Zealand physicist Ernest Rutherford discovered the proton. The discovery was based on the theory that hydrogen nucleus H+, as an elementary particle, is present in the nuclei of other atoms. So, he called this fundamental building block the “proton.” In fact, Rutherford suggested two terms, including proton and prouton, to highlight the role of William Prout’s discovery of the electron.
Finally, in 1932, another British physicist Sir James Chadwick announced the discovery of neutrons, proving that the gamma ray hypothesis was inaccurate. At this point, all three main components of an atom have been discovered.
What Is a Proton: The Definition
As we’ve discussed the discovery of protons, let’s explain what protons are and how they work.
Protons are stable subatomic particles that carry a positive electric charge and are found in the nucleus of an atom. The term “proton” means “first” in Greek and denotes the hydrogen nucleus.
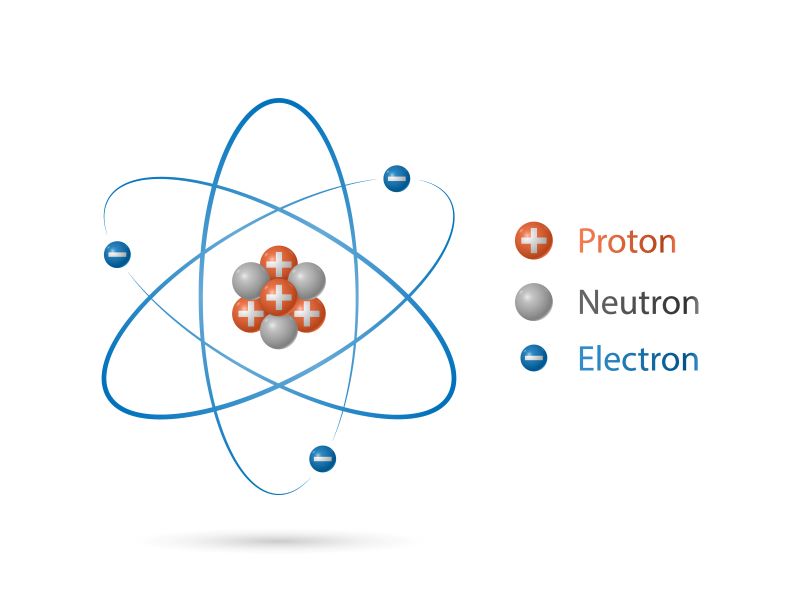
Besides, protons are one of three main subatomic particles that are located in each atom. The other two subatomic particles found in atoms are neutrons and electrons.
Protons are also made of particles called quarks. Any proton consists of two up quarks and one down quark that are held together by means of the strong force known as gluons.
Protons, Neutrons, and Electrons: What’s the Difference?
There are three main subatomic particles found in an atom, including protons, neutrons, and electrons. But what are the differences between them? Here’s the answer for you.
The main difference between protons, neutrons, and electrons is that protons are positively charged particles, electrons are negatively charged particles, and neutrons are neutral particles with no charge.
Read more about the properties of protons, neutrons, and electrons in this chapter on Chem LibreTexts.
Here’s what you need to know to easily differentiate between protons, neutrons, and electrons.
The Properties of Protons
Protons are subatomic particles with a positive electrical charge of one (+1). Besides, each proton has a mass of 1 atomic mass unit (amu), which is ~ 1.67×10-27kg.
Protons are found in the nucleus of an atom and are held together by means of the strong nuclear force. They are located in the center of an atom, which is often referred to as the dense region of the atom.
The Properties of Electrons
Electrons are subatomic particles with a negative electrical charge of one (-1). Electrons are much smaller than protons and neutrons and the mass of an electron is ~ 1/2000 of the mass of a proton or neutron.
Besides, unlike protons and neutrons, electrons don’t consist of smaller particles. In terms of location, electrons are found outside the nucleus and are constantly moving around it.
The Properties of Neutrons
While protons are positively charged and electrons carry a negative charge, neutrons are electrically neutral subatomic particles. Neutrons have almost the same diameter as protons and the mass of a neutron is slightly greater than 1 amu.
Just like protons, neutrons are found in the atomic nucleus and are held together by the strong nuclear force. Similarly to protons, each neutron consists of smaller particles. But in this case, there are two down quarks and one up quark in each neutron.
The Importance of Protons
The fact that protons are one of the three main subatomic particles makes their importance quite obvious. But what actually makes protons so useful?
The main reason why protons are important is that the number of protons in a given atom determines its elemental identity. Without protons and atomic numbers, we wouldn’t be able to differentiate different elements.
So, the reason why a carbon atom is a carbon atom is that it has 6 protons. An atom with 10 protons is neon and an atom with 24 protons is chromium. Evidently, the number of protons is what allows us to differentiate between elements.
In a nutshell, there would be no atoms without protons along with other subatomic particles. As a matter of fact, atoms combine to form molecules and molecules form substances or matter. So, without atoms, there would be no molecules and no matter. In simpler terms, we wouldn’t even exist.